Company Profile
PharmaEngine, Inc. began operations in February 2003. PharmaEngine is a networked pharmaceutical company that operates according to a "Virtual Pharmaceutical Company Business Model" model to focus on new drug development and lower related risks.
Business Scope
PharmaEngine focuses on oncology therapies. Our commercial product, ONIVYDE®, is a novel and stable encapsulated form of the marketed chemotherapy drug irinotecan in a long-circulating nanoliposome for the treatment of patients with metastatic adenocarcinoma of the pancreas. One of our R&D projects, PEP07, is a CHK1 inhibitor and is currently in Phase I clinical trial. Another R&D project, PEP08, is a PRMT5 inhibitor and is currently in Phase I clinical trial.
PharmaEngine continues to provide ONIVYDE® in the Taiwan market and establish a drug safety reporting system to ensure patient medication safety, while aggressively advancing clinical trials for PEP07 and PEP08, and developing other projects in our pipeline. We also strive to evaluate the licensing-in of new drug projects, using our extensive experience and expertise, to push the projects into clinical studies and commercialization.
Development Strategy
-
Adopt "Virtual Pharmaceutical Company Business Model" to
strengthen international strategic alliances and build an international R&D team -
Aggressively train R&D professionals to elevate new drug development techniques and build a sustainable business.
-
Our vision:
"Become the most professional and innovative new drug development company that specializes on oncology therapies in the world."
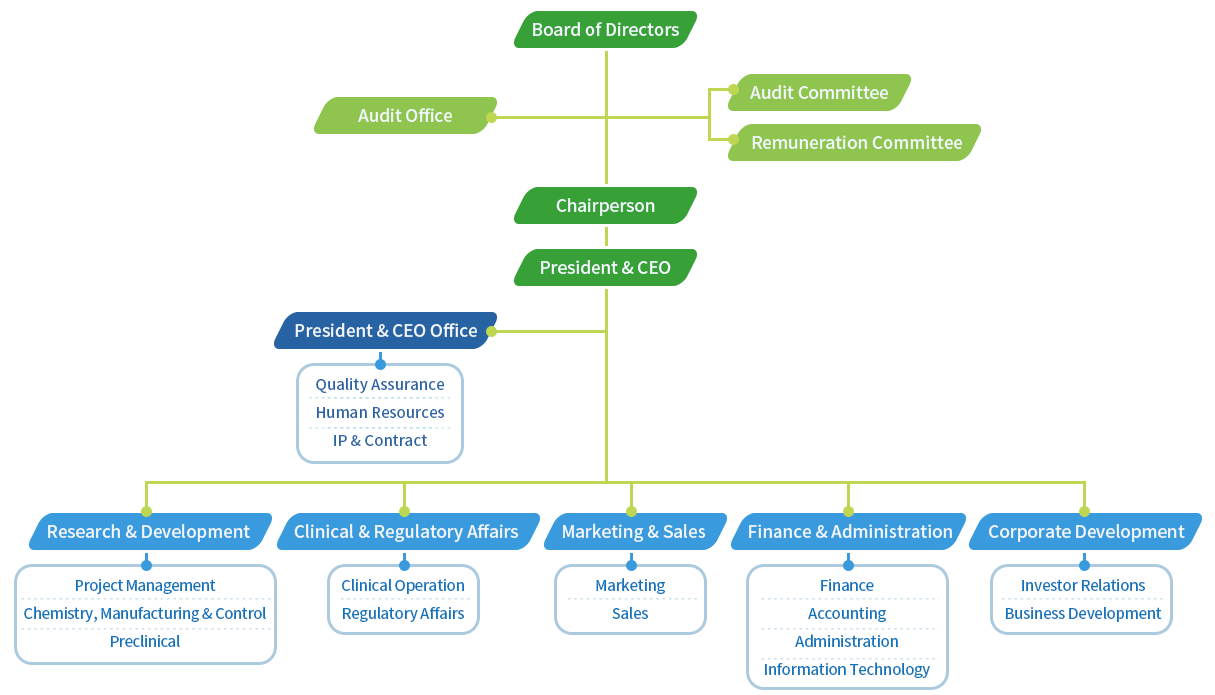
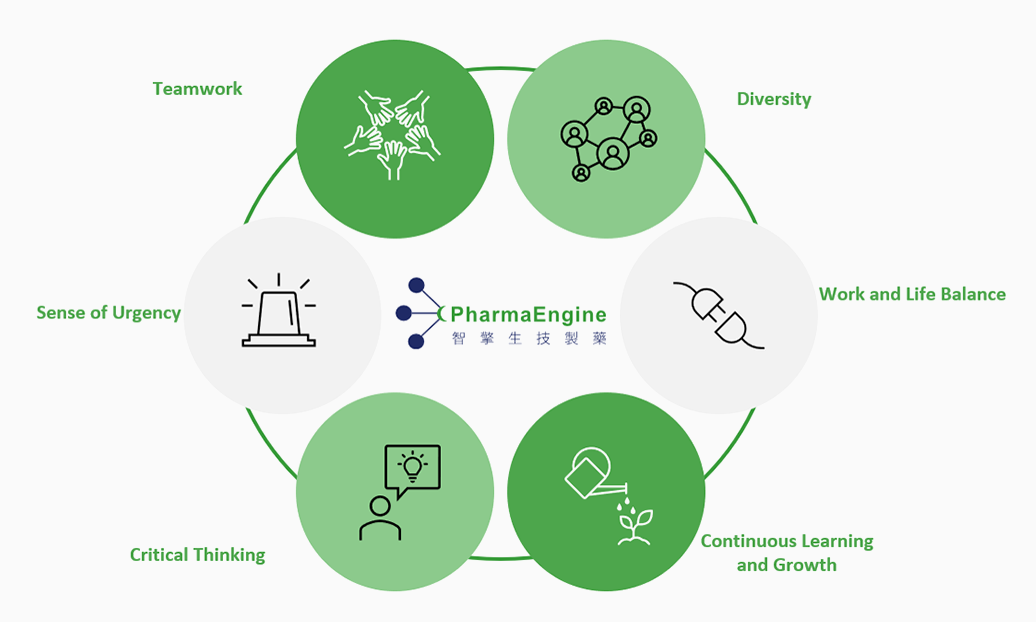
PharmaEngine promotes a warm company culture.
We believe these six core values are the key to a successful corporation.
We believe these six core values are the key to a successful corporation.
Strategic Partners
Let's talk partnership!
More
